Abstract
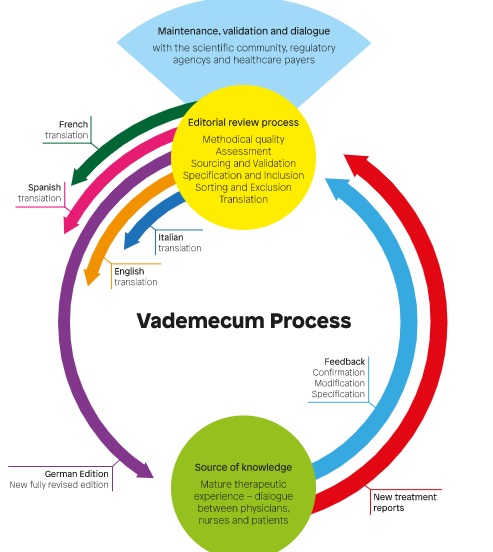
Background: therapy in whole medical systems involves a large number of medicinal products. One source of knowledge of clinical properties of such products is the experience of therapy providers. A systematic approach to documentation, assessment, and aggregation of physicians’ experiences with anthroposophic medicinal products (AMPs) has been developed: the Vademecum of Anthroposophic Medicines.
Material and Methods: the Vademecum contains structured information on AMPs, including therapeutic rationale, indications, and therapy recommendations. The information is based on a 17-item questionnaire of physicians’ therapy experiences, which is peer-reviewed by an interdisciplinary editorial board. We conducted a descriptive analysis of the Vademecum, 4th edition.
Results: the Vademecum comprised 799 different AMPs, used for 1,773 indications, based on 2,543 questionnaires submitted by 274 physicians from 19
countries. The 799 AMPs comprised 52.6% of all AMPs marketed in Germany in 2015–2016. The 1,773 indications corresponded to 544 different ICD-10 three-digit codes, amounting to 29.3% (n = 544/1,854) of all three-digit codes.
A total of 30.6% (n = 542/1,773) of indications were supported by ≥2 questionnaires.
Conclusions: the current Vademecum covers more than half of all AMPs, used for more than one fourth of all ICD-10 three-digit codes. The Vademecum approach may be relevant for medicinal products from other whole medical systems.
Leggi l’articolo